Shares of Santa Clara, CA-based XenoPort ($XNPT) were crushed today after the biotech touted what it called positive preliminary efficacy results from a midstage study of its oral psoriasis drug. While the biotech claimed that its drug is ready for Phase III, XenoPort faces a crowded set of late-stage rivals with a drug that posted some troubling side effects. And investors swiftly turned from upbeat to skeptical this morning as they had a chance to ponder the results, sending shares down 23% by midmorning.
The biotech, which has been earning a small amount of revenue from its disappointing restless leg syndrome drug Horizant, believes that its fumarate pill could carve into the increasingly crowded psoriasis market with a once-daily oral offering that might allow a better safety profile.
Investigators used the 12-week trial to test XP23829 against a placebo on the commonly used Psoriasis Area and Severity Index (PASI) score, scoring reductions for a once-daily 800-mg pill that almost doubled a placebo response and did significantly better at 400 mg (-38% on PASI compared to a PASI reduction of -25% for the placebo). The twice-daily 400-mg dose also edged the single, daily 800-mg pill.
"We believe these clinical data demonstrate for the first time that a (monomethyl fumarate) prodrug other than dimethyl fumarate can be effective in reducing lesions in psoriatic patients," noted XenoPort CMO Richard Kim in a statement. "The magnitude of XP23829's effect on the primary efficacy endpoint met our expectations for this relatively short duration trial and we are particularly encouraged by the results with 800-mg once-daily dosing. Based on what is known about fumarates, we believe that the efficacy of XP23829 is likely to improve with a more extended duration of treatment beyond 12 weeks." Kim also claimed that the safety profile was beneficial and that the results might "read through" to multiple sclerosis.
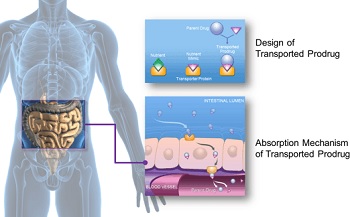 |
| Graphic depicting the absorption mechanism of XenoPort's oral platform--Courtesy of XenoPort |
Detailing the side effects of this drug, though, investigators noted 22% to 40% of the patients in the drug arms suffered from diarrhea. "There were two treatment emergent serious adverse events assessed as possibly related to treatment with XP23829: acute cholecystitis and enterocolitis. Both subjects recovered." There were also significantly higher rates of abdominal pain and vomiting in the 800-mg arm. And one in 20 patients had grade 2 lymphopenia--low levels of protective lymphocytes in the blood--with 15% in the grade 1 category.
Analysts weren't in a forgiving mood today.
"While the drug demonstrated clear activity, we think neither its efficacy or safety profile appear compelling or differentiated. We do not believe further development of '829 is warranted," noted Cowen's Eric Schmidt.
XenoPort is a late arrival in the most recent psoriasis drug development race. Novartis is already well in front with its IL-17 program for secukinumab, an injectable drug approved in January as Cosentyx. Eli Lilly ($LLY) has also been racking up positive late-stage studies for its IL-17-blocking ixekizumab, trailed by Merck's ($MRK) MK-3222 and Johnson & Johnson's ($JNJ) IL-23 inhibitor guselkumab. AstraZeneca ($AZN) had been among the leaders with brodalumab, until Amgen ($AMGN) dumped their partnership based on the marketing troubles it predicted post-Phase III based on suicidal thinking. Then AstraZeneca followed, outlicensing the drug to Valeant, which has a rep for taking on controversial products.
Trying to fit a new, branded oral drug into the race won't be easy. But XenoPort says that it can follow a straight route into late-stage studies, which will have to be much larger than the Phase II to satisfy regulators.
- here's the release