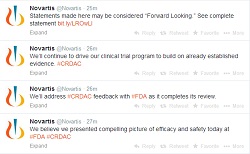
 |
| Novartis used Twitter to comment throughout its FDA panel meeting on serelaxin (click to expand). |
After a daylong session heavy on scathing criticism, a panel of FDA advisers voted unanimously against approving Novartis' ($NVS) in-development heart drug serelaxin, casting serious doubts on the treatment's potential.
In an 11-0 vote, the agency's Cardiovascular and Renal Drugs Advisory Committee struck down Novartis' case for the heart failure treatment, taking the drugmaker to task over the design of its 1,161-patient pivotal trial, gaps in the provided data and serious questions over serelaxin's efficacy. Echoing a particularly harsh review from FDA staff, the panel said Novartis failed to confirm the drug's effect on shortness of breath and the rate of heart failure.
The agency is not required to follow the recommendations of its advisers, though it usually does, and the FDA is expected to make a final decision on serelaxin by May 17.
Novartis said in a statement that it stands by its Phase II and III results, and, in what is perhaps a first in biopharma, the company's corporate Twitter account chimed in throughout the panel meeting to say the same thing. After the unanimously negative vote, Novartis tweeted that it believes investigators "presented (a) compelling picture of efficacy and safety," and the drugmaker plans to address the panel's feedback with the FDA. "We'll continue to drive our clinical trial program to build on already established evidence," the company tweeted.
If serelaxin's Phase III data aren't enough to convince the FDA, regulators may hold out for results from a larger outcomes trial, which aren't expected until at least 2016, Novartis has said.
Just last year, serelaxin was among the first drugs imbued with the FDA's breakthrough therapy designation, guaranteeing it a front-row seat with regulators and a speedy review process. Novartis long touted the drug as a blockbuster in the making, expecting sales to peak well above $1 billion, but many analysts remained cautious, citing some mixed results in mid- and late-stage studies.
And things began to come apart in January when the European Medicines Agency's Committee for Medicinal Products for Human Use recommended against approving serelaxin, pre-echoing the FDA panel's concerns and likely derailing Novartis' near-term plans for a European launch.
- here's Novartis' statement